Capabilities of positron emission tomography/computed tomography in a comparative assessment of the effect of various targeted therapy options in patients with EGFR-mutated non-small-cell lung cancer
- Authors: Strutynsky V.A.1,2, Sinitsyn V.E.1, Platonova O.E.2
-
Affiliations:
- Lomonosov Moscow State University
- JSC “Medicine”
- Issue: Vol 5, No 3 (2024)
- Pages: 394-406
- Section: Original Study Articles
- URL: https://bakhtiniada.ru/DD/article/view/310026
- DOI: https://doi.org/10.17816/DD624504
- ID: 310026
Cite item
Full Text
Abstract
BACKGROUND: No publications in the Russian medical literature have examined the potential of positron emission tomography combined with computed tomography through a comparative evaluation of the effect of various targeted therapies involving tyrosine kinase inhibitors in patients with non-small cell lung cancer and mutations in the EGFR gene.
AIM: To explore the capabilities of positron emission tomography combined with computed tomography based on the RECIST 1.1 criteria and changes in SUVmax and SUVmean metabolic parameters for the comparative assessment of the tumor response to targeted monotherapy and combination therapy with tyrosine kinase inhibitors in patients with EGFR-mutant non-small cell lung cancer.
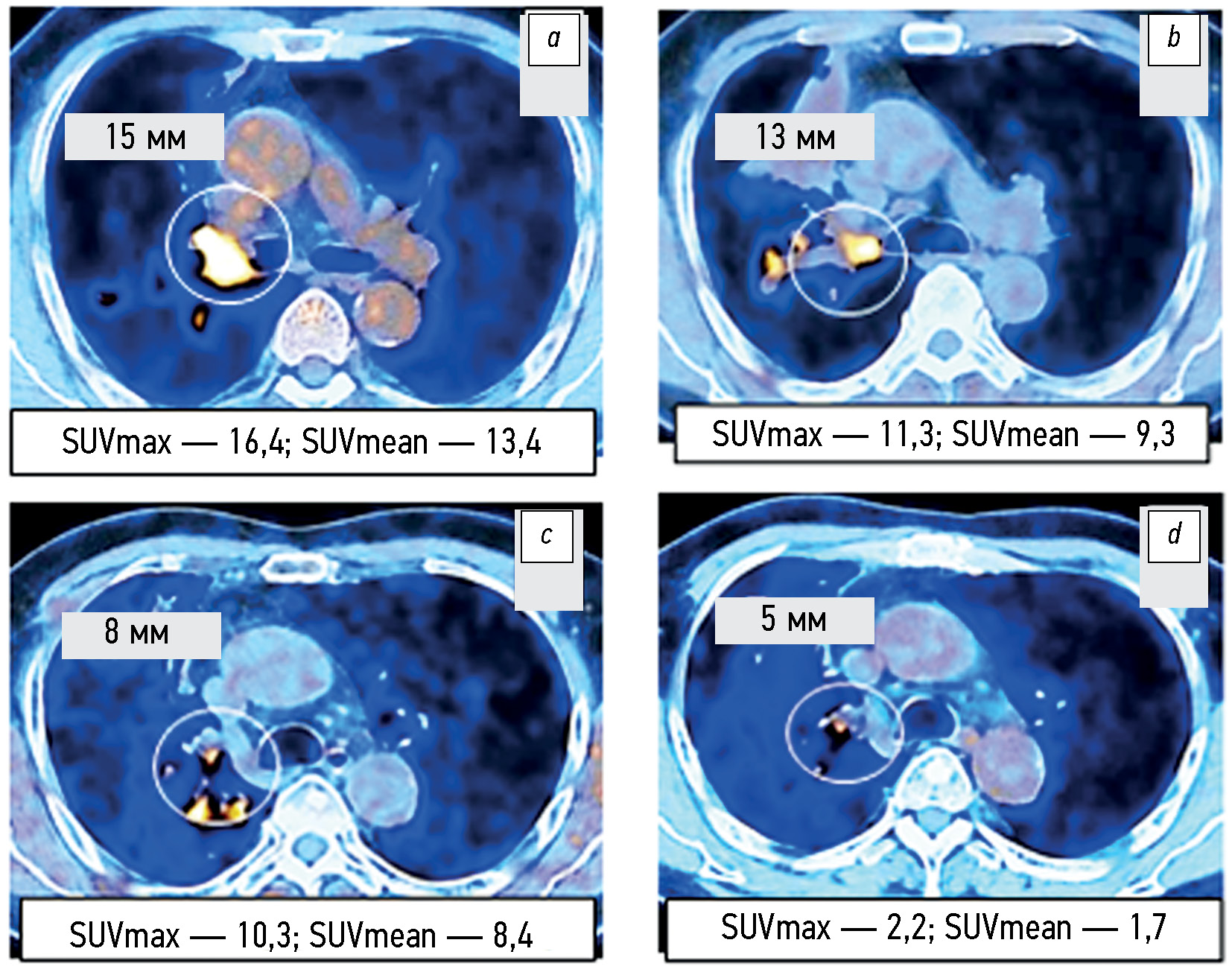
MATERIALS AND METHODS: The 2019–2022 examination records of positron emission tomography combined with computed tomography with 18F-fluorodeoxyglucose (18F-FDG) in 105 patients with non-small cell lung cancer were analyzed, including 75 patients with EGFR-activating mutation. The radiation exposure was adjusted individually and ranged from 45 to 90 mSv. The volume activity of the 18F-FDG radiopharmaceutical was 260–500 MBq. The change in the total largest diameters of the target lesions and SUVmax and SUVmean metabolic parameters were assessed before treatment initiation and 1.5–2.0 months after it. The follow-up duration for the changes in positron emission tomography combined with computed tomography findings in 17 patients with non-small cell lung cancer was at least 12 months.
RESULTS: According to positron emission tomography combined with computed tomography images and SUVmax and SUVmean changes, disease progression was significantly less common (p = 0.043 and p =0.029) in patients with EGFR-mutant non-small cell lung cancer from Groups 2 and 3 who received combination therapy with tyrosine kinase inhibitors and bevacizumab or chemotherapy than in Group 1 and control group (4.2% vs. 20.0%–21.8%). An insignificant trend (p =0.092) to a higher partial response to therapy (58.3% vs. 40.0%) was noted. Similar changes in the total largest diameters of the target lesions at the early stage of therapy appeared to be not significant (p =0.187). Within the long-term follow-up of some patients with non-small cell lung cancer, in at least 50% of cases, changes in the total largest lesion diameters are consistent with the relevant SUVmax and SUVmean alterations found in the first control study.
CONCLUSIONS: Based on the positron emission tomography combined with computed tomography data and alterations in SUVmax and SUVmean metabolic parameters, the early tumor response to combined therapy with tyrosine kinase inhibitors and bevacizumab or chemotherapy compared with targeted monotherapy with tyrosine kinase inhibitors or chemotherapy of the control group was characterized by a significantly lower rate of metabolic disease progression, although a similar tendency of the change in the total largest diameters of target lesions according to RECIST 1.1. was not significant. Changes in SUVmax and SUVmean metabolic parameters at the early stage of therapy are at least 50% faster than similar changes in the total largest diameters of the target lesions, which can be used for the timely identification of patients with a high risk of further progression as determined by RECIST 1.1.
Full Text
##article.viewOnOriginalSite##About the authors
Vladislav A. Strutynsky
Lomonosov Moscow State University; JSC “Medicine”
Author for correspondence.
Email: Rammen2@yandex.ru
SPIN-code: 6810-5644
Russian Federation, Moscow; Moscow
Valentin E. Sinitsyn
Lomonosov Moscow State University
Email: Vsini@mail.ru
ORCID iD: 0000-0002-5649-2193
SPIN-code: 8449-6590
MD, Dr. Sci. (Medicine), Professor
Russian Federation, MoscowOksana E. Platonova
JSC “Medicine”
Email: Platonova@medicina.ru
ORCID iD: 0000-0003-0093-7285
MD, Cand. Sci. (Medicine)
Russian Federation, MoscowReferences
- Kaprin AD, Starinskii VV, Shakhzadova AO. Malignant neoplasms in Russia in 2019 (morbidity and mortality). Moscow: Moskovskii gosudarstvennyi nauchno–issledovatel’skii meditsinskii institut im. P.A. Gertsena; 2020. (In Russ.)
- Sakaeva DD, Reutova EV. Targeted therapy for metastatic non-small cell lung cancer. In: Laktionov KK, Breder VV, editors. Lung cancer. Moscow: “Granat”; 2020. P:75–88. (In Russ.)
- Tyulyandin SA. Targeted therapy: twenty years of success and failures. Practical oncology. 2019;20(4):274–288. doi: 10.31917/2004274
- Deng W, Wang K, Jiang Y, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-small-cell lung cancer: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2022;12(8):e062036. doi: 10.1136/bmjopen-2022-062036
- Landre T, Des Guetz G, Chouahnia R, et al. First-line angiogenesis inhibitor plus erlotinib versus erlotinib alone for advanced non-small-cell lung cancer harboring an EGFR mutation. Cancer Res Clin Oncol. 2020;146(12):3333–3339. doi: 10.1007/s00432-020-03311-w
- Maemondo M, Fukuhara T, Saito H, et al. NEJ026: Final overall survival analysis of bevacizumab plus erlotinib treatment for NSCLC patients harboring activating EGFR-mutations. Journal of Clinical Oncology. 2020;38(15):9506–9506. doi: 10.1200/JCO.2020.38.15_suppl.9506
- Rocco D, Della Gravara L, Palazzolo G, et al. The role of antiangiogenic monoclonal antibodies combined to EGFR-TKIs in the treatment of advanced non-small cell lung cancer with activating EGFR mutations: acquired resistance mechanisms and strategies to overcome them. Cancer Drug Resist. 2022;5(4):1016–1024. doi: 10.20517/cdr.2022.77
- Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625–635. doi: 10.1016/S1470-2045(19)30035-X
- Stinchcombe TE, Jänne PA, Wang X, et al. Effect of Erlotinib Plus Bevacizumab vs Erlotinib Alone on Progression-Free Survival in Patients With Advanced EGFR-Mutant Non-Small Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5(10):1448–1455. doi: 10.1001/jamaoncol.2019.1847
- Meshcheryakova NA. Positron emission tomography combined with computed tomography in the diagnosis and evaluation of treatment effectiveness of non-small cell lung cancer [dissertation]. Moscow; 2018.
- Meshcheryakova NA, Dolgushin MB, Borisova TN, Davydov MM, Laktionov KK. Efficacy of 18F-FDG and 18F-FLT PET/CT for Assessment of Chemoradiotherapy in Patient with Non-Small Cell Lung Cancer (Clinical Observation). Medical Visualization. 2017;(1):53–56. doi: 10.24835/1607-0763-2017-1-53-56
- Wahl R, Jacene Н, Kasamon Y, Lodge M. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J Nucl Med. 2009;50(5):4–11. doi: 10.2967/jnumed.111.093443
- Gelezhe PB, Morozov SP, Shavladze N. Comparison of the accuracy of evaluating the attenuation correction and tumor size during sequential performance of breast 18F-FDG PET CT and PET/ MRI. Vestnik of the Russian scientific center of roentgenoradiology. 2019;19(4):48–62. EDN: LWGHUU
- Ding Q, Chen X, Yang L, et al. PET/CT evaluation of response to chemotherapy in non-small cell lung cancer: PET response criteria in solid tumors (PERCIST) versus response evaluation criteria in solid tumors (RECIST). J Thorac Dis. 2014;6(6):677–683. doi: 10.3978/j.issn.2072-1439.2014.05.10
- Pierson C, Grinchak T, Sokolovic C, et al. Response criteria in solid tumors (PERCIST/RECIST) and SUVmax in early-stage non-small cell lung cancer patients treated with stereotactic body radiotherapy. Radiat Oncol. 2018;13(1):34. doi: 10.1186/s13014-018-0980-7
- Beer L, Hochmair M, Haug AR, et al. Comparison of RECIST and PERCIST for the Evaluation of Response to PD-1/PD-L1 Blockade Therapy in Patients With Non-Small Cell Lung Cancer. Clin Nucl Med. 2019;44(7):535–543. doi: 10.1097/RLU.0000000000002603
- Kaira K, Higuchi T, Naruse I, et al. Metabolic activity by 18 F–FDG-PET/ CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging. 2018;45(1):56–66. doi: 10.1007/s00259-017-3806-1
- Khodzhibekova MM. Value of combined PET/CT in the diagnosis and monitoring of treatment of lymphoma patients [dissertation]. Moscow; 2019. (In Russ.)
- Koopman D, Jager PL, Slump CH, et al. SUV variability in EARL-accredited conventional and digital PET. EJNMMI Res. 2019;9(1):106. doi: 10.1186/s13550-019-0569-7
- Xie X, Chen H, Yang H, Lin H. Predictive value of positron emission tomography for the prognosis of molecularly targeted therapy in solid tumors. Z Onco Targets Ther. 2018;7(11):8885–8899. doi: 10.2147/OTT.S178076
- Kamiyoshihara M, Igai H, Ohsawa F, Numajiri K, et al. Gefitinib Monotherapy Afforded Long-Term Survival of an Octogenarian Patient with a Postoperative Recurrence of a Pulmonary Adenocarcinoma — A Case Report. Gan To Kagaku Ryoho. 2023;50(2):187–189. (In Japanese)
- Stinchcombe TE. Foreword: Gefitinib in Non-Small-Cell Lung Cancer: The IDEAL 1 Trial. J Clin Oncol. 2023;41(6):1159–1160. doi: 10.1200/JCO.22.02660
- Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19(1):139–148. doi: 10.1016/S1470-2045(17)30729-5
- Liu M, Luo N, Fang Z, Liu Q, et al. The efficacy and toxicity of maintenance therapy with bevacizumab plus pemetrexed versus bevacizumab/pemetrexed alone for stage IIIB/IV nonsquamous non-small cell lung cancer: A meta-analysis of randomized controlled trials. Clin Pharm Ther. 2022;47(2):157–167. doi: 10.1111/jcpt.13534
- Chen Z, Shen S. Intercalated combination of chemotherapy and erlotinib for stage IIIA non-small-cell lung cancer: a multicenter, open-label, single-arm, phase II study. Cancer Manag Res. 2019;11:6543–6552. doi: 10.2147/CMAR.S189287 DOI: https://doi.org/10.17816/DD624504
Supplementary files