Structural gray matter changes in primary progressive aphasia variants
- Authors: Akhmadullina D.R.1, Konovalov R.N.1, Shpilyukova Y.A.1, Fedotova E.Y.1
-
Affiliations:
- Research Center of Neurology
- Issue: Vol 4, No 4 (2023)
- Pages: 467-480
- Section: Original Study Articles
- URL: https://bakhtiniada.ru/DD/article/view/262949
- DOI: https://doi.org/10.17816/DD567783
- ID: 262949
Cite item
Abstract
BACKGROUND: Primary progressive aphasia is a rare neurodegenerative disease with high clinical, genetic, and pathomorphological heterogeneity that greatly complicates its diagnosis. Voxel-based morphometry can be used to objectively assess structural gray matter changes and determine atrophy patterns in variants of primary progressive aphasia, which can improve the diagnosis and our understanding of its pathogenesis.
AIMS: This study aimed to evaluate the patterns of atrophy in each of the primary progressive aphasia variants in comparison with the control group.
MATERIALS AND METHODS: Patients diagnosed with one of the primary progressive aphasia variants, established in accordance with the current diagnostic criteria, were included in the main group. The control group consisted of healthy volunteers without any neurological symptoms or structural brain changes. All participants underwent brain magnetic resonance imaging. The obtained images were processed and used for voxel-based morphometry, which was performed by comparing the gray matter volume between each of the primary progressive aphasia variants and the control group. The study was adjusted for the sex, age, and intracranial volume of the participants.
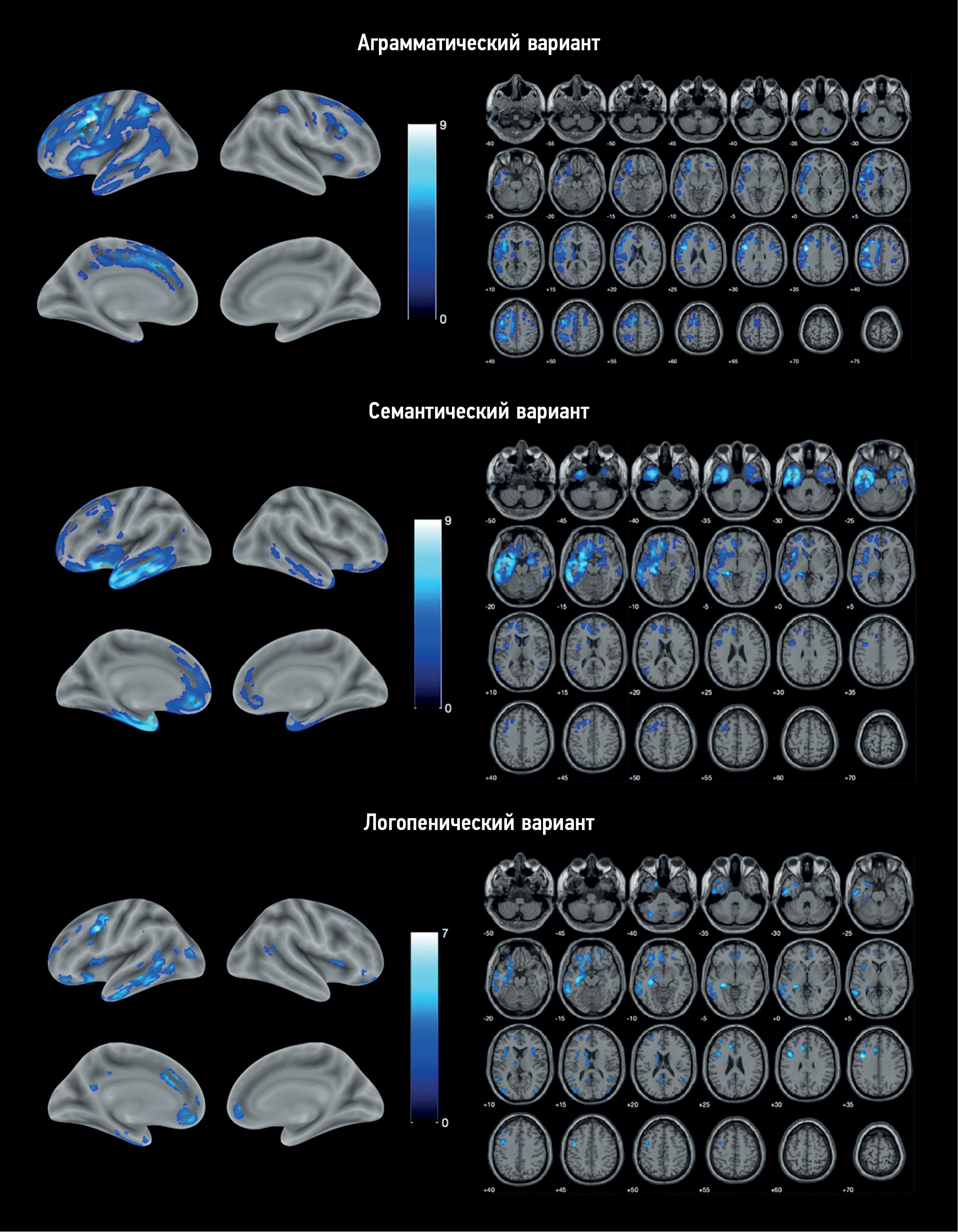
RESULTS: The study enrolled 25 patients with nonfluent, 11 with semantic, and 9 with logopenic variants of primary progressive aphasia, as well as 20 healthy volunteers. Voxel-based morphometry showed a specific atrophy pattern in each of the variants of primary progressive aphasia, with predominant involvement of the frontal and insular lobes in nonfluent, temporal lobe and hippocampus in semantic, and a more diffuse frontotemporal pattern in logopenic variants.
CONCLUSIONS: The study revealed gray matter atrophy patterns specific to each variant of primary progressive aphasia. The obtained results mainly correspond to the clinical presentations of the disease. Moreover, some findings (e.g., absence of the posterior perisylvian atrophy and reduced motor cortex volume in the logopenic variant, atrophy of the orbitofrontal cortex and cerebellum in the nonfluent variant, and premotor cortex, precentral, and inferior frontal gyrus degeneration in the semantic variant) do not correlate with the usual understanding of primary progressive aphasia pathogenesis and require further study.
Full Text
##article.viewOnOriginalSite##About the authors
Diliara R. Akhmadullina
Research Center of Neurology
Author for correspondence.
Email: akhmadullinadr1@gmail.com
ORCID iD: 0000-0001-6491-2891
SPIN-code: 5721-8567
Russian Federation, Moscow
Rodion N. Konovalov
Research Center of Neurology
Email: krn_74@mail.ru
ORCID iD: 0000-0001-5539-245X
SPIN-code: 2515-7673
Scopus Author ID: 23497502900
ResearcherId: B-6834-2012
MD, Cand. Sci. (Med.)
Russian Federation, MoscowYulia A. Shpilyukova
Research Center of Neurology
Email: jshpilyukova@gmail.com
ORCID iD: 0000-0001-7214-583X
SPIN-code: 7502-8984
MD, Cand. Sci. (Med.)
Russian Federation, MoscowEkaterina Y. Fedotova
Research Center of Neurology
Email: ekfedotova@gmail.com
ORCID iD: 0000-0001-8070-7644
SPIN-code: 3466-2212
MD, Dr. Sci. (Med.)
Russian Federation, MoscowReferences
- Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011; 76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6
- Bisenius S, Neumann J, Schroeter ML. Validating new diagnostic imaging criteria for primary progressive aphasia via anatomical likelihood estimation meta-analyses. European Journal of Neurology. 2016;23(4):704–712. doi: 10.1111/ene.12902
- Lombardi J, Mayer B, Semler E, et al. Quantifying progression in primary progressive aphasia with structural neuroimaging. Alzheimer’s & Dementia. 2021;17(10):1595–1609. doi: 10.1002/alz.12323
- Chapman CA, Polyakova M, Mueller K, et al. Structural correlates of language processing in primary progressive aphasia. Brain Communications. 2023;5(2). doi: 10.1093/braincomms/fcad076
- Canu E, Agosta F, Battistella G, et al. Speech production differences in English and Italian speakers with nonfluent variant PPA. Neurology. 2020;94(10):e1062–e1072. doi: 10.1212/WNL.0000000000008879
- Akhmadullina D, Konovalov R, Shpilyukova Y, et al. Brain atrophy patterns in patients with frontotemporal dementia: voxel-based morphometry. Bulletin of Russian State Medical University. 2020;(6):84–89. doi: 10.24075/brsmu.2020.075
- Lampe L, Huppertz HJ, Anderl-Straub S, et al. Multiclass prediction of different dementia syndromes based on multi-centric volumetric MRI imaging. NeuroImage: Clinical. 2023;37:103320. doi: 10.1016/j.nicl.2023.103320
- Staffaroni AM, Ljubenkov PA, Kornak J, et al. Longitudinal multimodal imaging and clinical endpoints for frontotemporal dementia clinical trials. Brain. 2019;142(2):443–459. doi: 10.1093/brain/awy319
- zenodo.org [Internet]. spunt/bspmview: BSPMVIEW v.20161108 (Version 20161108). Zenodo. [cited 26 July 2023]. Available from: https://zenodo.org/badge/latestdoi/21612/spunt/bspmview doi: 10.5281/zenodo.168074
- Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55(3):335–346. doi: 10.1002/ana.10825
- Tetzloff KA, Utianski RL, Duffy JR, et al. Quantitative analysis of agrammatism in agrammatic primary progressive aphasia and dominant apraxia of speech. Journal of Speech, Language, and Hearing Research. 2018;61(9):2337–2346. doi: 10.1044/2018_JSLHR-L-17-0474
- Whitwell JL, Duffy JR, Strand EA, et al. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: An MRI and FDG-PET study. Brain and Language. 2013;125(3):245–252. doi: 10.1016/j.bandl.2013.02.005
- Mandelli ML, Vitali P, Santos M, et al. Two insular regions are differentially involved in behavioral variant FTD and nonfluent/agrammatic variant PPA. Cortex. 2016;74:149–157. doi: 10.1016/j.cortex.2015.10.012
- Cordella C, Quimby M, Touroutoglou A, et al. Quantification of motor speech impairment and its anatomic basis in primary progressive aphasia. Neurology. 2019;92(17):e1992–e2004. doi: 10.1212/WNL.0000000000007367
- Breining BL, Faria AV, Tippett DC, et al. Association of Regional Atrophy With Naming Decline in Primary Progressive Aphasia. Neurology. 2023;100(6):e582–e594. doi: 10.1212/WNL.0000000000201491
- Rogalski E, Cobia D, Harrison TM, et al. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011;76(21):1804–1810. doi: 10.1212/WNL.0b013e31821ccd3c
- Samra K, MacDougall AM, Bouzigues A, et al. Genetic forms of primary progressive aphasia within the GENetic Frontotemporal dementia Initiative (GENFI) cohort: comparison with sporadic primary progressive aphasia. Brain Communications. 2023;5(2). doi: 10.1093/braincomms/fcad036
- Rohrer JD, Nicholas JM, Cash DM, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. The Lancet Neurology. 2015;14(3):253–262. doi: 10.1016/S1474-4422(14)70324-2
- McKenna MC, Li Hi Shing S, Murad A, et al. Focal thalamus pathology in frontotemporal dementia: Phenotype-associated thalamic profiles. Journal of the Neurological Sciences. 2022;436:120221. doi: 10.1016/j.jns.2022.120221
- Ziegler W, Ackermann H. Subcortical Contributions to Motor Speech: Phylogenetic, Developmental, Clinical. Trends in Neurosciences. 2017;40(8):458–468. doi: 10.1016/j.tins.2017.06.005
- Migliaccio R, Boutet C, Valabregue R, et al. The Brain Network of Naming: A Lesson from Primary Progressive Aphasia. PLOS ONE. 2016;11(2):e0148707. doi: 10.1371/journal.pone.0148707
- Wisse LEM, Ungrady MB, Ittyerah R, et al. Cross-sectional and longitudinal medial temporal lobe subregional atrophy patterns in semantic variant primary progressive aphasia. Neurobiology of Aging. 2021;98:231–241. doi: 10.1016/j.neurobiolaging.2020.11.012
- Fittipaldi S, Ibanez A, Baez S, et al. More than words: Social cognition across variants of primary progressive aphasia. Neuroscience & Biobehavioral Reviews. 2019;100:263–284. doi: 10.1016/j.neubiorev.2019.02.020
- Brown JA, Deng J, Neuhaus J, et al. Patient-Tailored, Connectivity-Based Forecasts of Spreading Brain Atrophy. Neuron. 2019;104(5):856–868.e5. doi: 10.1016/j.neuron.2019.08.037
- Collins JA, Montal V, Hochberg D, et al. Focal temporal pole atrophy and network degeneration in semantic variant primary progressive aphasia. Brain. 2017;140(2):457–471. doi: 10.1093/brain/aww313
- Kumfor F, Landin-Romero R, Devenney E, et al. On the right side? A longitudinal study of left- versus right-lateralized semantic dementia. Brain. 2016;139(3):986–998. doi: 10.1093/brain/awv387
- Henry ML, Wilson SM, Babiak MC, et al. Phonological Processing in Primary Progressive Aphasia. Journal of Cognitive Neuroscience. 2016;28(2):210–222. doi: 10.1162/jocn_a_00901
- Montembeault M, Brambati SM, Gorno-Tempini ML, Migliaccio R. Clinical, Anatomical, and Pathological Features in the Three Variants of Primary Progressive Aphasia: A Review. Frontiers in Neurology. 2018;9. doi: 10.3389/fneur.2018.00692
- Bergeron D, Gorno-Tempini ML, Rabinovici GD, et al. Prevalence of amyloid-β pathology in distinct variants of primary progressive aphasia. Annals of Neurology. 2018;84(5):729–740. doi: 10.1002/ana.25333
- Preiß D, Billette OV, Schneider A, et al. The atrophy pattern in Alzheimer-related PPA is more widespread than that of the frontotemporal lobar degeneration associated variants. NeuroImage: Clinical. 2019;24:101994. doi: 10.1016/j.nicl.2019.101994
Supplementary files